West Nile virus (Orthoflavivirus nilense) is a seasonal mosquito-borne disease which occurs in Saskatchewan and many other Canadian provinces. It was introduced into New York City in 1999 and spread across North America over several years. The Western/Northern region of the Canadian Wildlife Health Cooperative, which provides diagnostic services to Saskatchewan, the Northwest Territories, and Yukon, has seen a significant upswing in WNV positive wild birds from Saskatchewan this year. Questions from submitters and the desire to provide a resource on the disease have led to writing this article.
Key facts:
- West Nile Virus was introduced into New York City in 1999 and spread across North America over several years
- West Nile Virus (WNV) is spread by mosquitoes, primarily those of the genus Culex
- WNV is maintained in nature in a mosquito-bird-mosquito transmission cycle
- Transmission to humans, horses, other mammals, and birds occurs mainly through the bites of infected mosquitoes
- There is no evidence that direct, casual contact with birds transmits the virus
- Birds: infection is frequently fatal for birds – corvids (ravens, crows, magpies, jays) and raptors (eagles, hawks, falcons, owls) are especially susceptible to the virus; survivors may suffer from lasting effects
- Horses: infection rarely causes illness; when clinical disease occurs, presentation can vary from fever and depression to severe neurological signs and death; long-term effects are possible; effective vaccines are available
- Humans: infection often causes no symptoms, but when symptoms appear they can vary from flu-like illness to (uncommonly) neurological signs and rarely death; long-term effects are possible; a vaccine is not available
Current Status in Saskatchewan
As of September 12th, 2025, 83 wild birds in Saskatchewan have tested positive for WNV, with 2 additional cases undergoing further testing to confirm infection. Our lab started
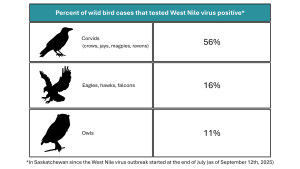
testing wild birds for WNV at the beginning of May, with the first positives detected at the end of July. Since the first positive test results, 56% of corvids tested were positive, as were 16% of raptors (not including owls), and 11% of owls1. Over 300 birds from Saskatchewan have been tested to date.
As of September 12th, 2025, the risk of WNV transmission in the prairie ecozone of Saskatchewan is high, with a moderate risk level in the boreal transition ecoregion, and minimal risk further north.
For up-to-date information on WNV risk level and bird, horse, and human surveillance results in Saskatchewan, visit the Government of Saskatchewan West Nile Virus Risk Level and Surveillance Results webpage.
1These results are not a representation of the prevalence of WNV infection in wild birds but reflect the percentage of deaths that can likely be attributed to the virus.
Transmission
Mosquitoes are the primary method by which the virus is spread. They acquire the virus when feeding on an infected bird and later spread it by feeding on other birds. But it’s not that simple.
Once a bird is infected, the virus replicates to levels that allow for transmission to other mosquitoes during a blood meal – like fishing, where you are more likely to get a catch if there are lots of fish in the lake. The infectious stage of a bird is dependent on the bird species (some are better than others for generating high virus levels) and generally lasts several days.
When a mosquito does pick up the virus, the virus must replicate again and move to the mosquito’s salivary glands for it to be able to transmit the disease. Therefore, the chance of a mosquito acquiring the virus and spreading disease is influenced by the bird species, the viral concentration in the bird’s blood, the mosquito species (not all species can become infected and different species prefer feeding on different hosts), and the ability of the virus to replicate inside of the mosquito. It is believed that WNV does not cause disease or death in mosquitoes themselves.
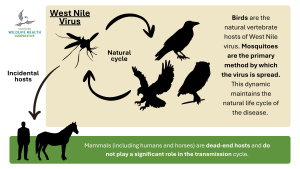
Mammals (including humans) are dead-end hosts and do not play a significant role in the transmission cycle. In mammals, the virus cannot increase its levels to a point where it can be picked up by other mosquitoes during subsequent blood meals. Although additional rare transmission pathways exist (such as from mother to infant or through open wound contact with infected human or avian blood), mosquitoes are the most important route. There is no evidence of the disease spreading through direct, casual contact with birds.
Given these transmission factors, what happens to the virus in the winter if outbreaks can occur every year? Mosquitoes are cold-blooded insects that require warm temperatures to increase and maintain their activity levels, metabolism, and development. Culex species mosquitoes enter a period of suspended development when environmental conditions are unfavorable (e.g. cold temperatures) and become active again when conditions improve (e.g. warmer temperatures). Female mosquitoes can also transmit the virus to her offspring (vertical transmission). As WNV does not harm the infected mosquito, the virus can survive through winter and spring until temperatures are warm enough for mosquito activity and virus transmission to resume, leading to some WNV occurrences in early summer with a peak in late summer and fall. Virus transmission ceases when mosquito activity stops once temperatures decrease or after a hard frost.
Signs and Symptoms
The time from infection until signs are seen (incubation period) is similar in birds, horses, and people – about 2 days to 2 weeks.
Birds
In some birds, WNV may cause few signs, such as in robins, but in others it can harm a wide range of tissues and systems including the central nervous system (see Figure 3 for a non-exhaustive list of clinical signs). Birds that survive WNV may suffer lasting effects such as damage to the brain, heart, and immune system that could have profound impacts on survival. Corvids, especially juveniles, are highly susceptible to the virus and most die within three weeks of infection.
WNV in birds needs to be confirmed with laboratory testing as several other illnesses can cause similar clinical signs, including avian influenza, avian cholera, and lead poisoning.
Horses
Clinical disease in horses is uncommon and generally mild but may progress to neurologic disease which can be severe and lead to death (see Figure 3 for a non-exhaustive list of clinical signs).
Many horses fully recover from infection with the virus, though some may exhibit lasting effects, including gait and behavioral abnormalities. Around a third of horses presenting with clinical signs die or require euthanasia due to complications. Individuals more at risk for death include foals (1 year or younger), older horses (15 years or older), those that present with recumbency and/or more serious clinical signs (e.g. seizures, coma), and unvaccinated horses.
Other illnesses cause similar clinical signs such as equine herpesvirus and rabies; a blood test is needed to confirm the diagnosis of WNV in live horses.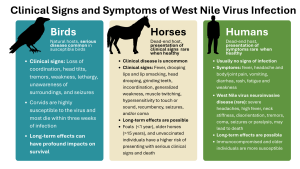
Humans
Serious disease in humans is relatively rare – around 80% of those who get infected will not experience symptoms. Those who do get sick can develop fever, headache and body/joint pain, vomiting, diarrhea, rash, fatigue, and weakness. Full recovery depends on the severity of symptoms and treatment provided, with some people suffering long-term effects such as muscle weakness/ache and fatigue.
Less than 1% of infected people (immunocompromised and older people being more susceptible) develop severe infection of the brain and more serious long-term effects. This can present with severe headaches, high fever, neck stiffness, disorientation, coma, tremors, seizures or paralysis, and may lead to death.
If you suspect West Nile virus disease, contact 811 or your healthcare provider for further assistance. Diagnosis is confirmed with blood tests.
Treatment
There is no specific treatment for WNV infection, meaning that there is no antiviral medication which specifically targets or is effective for the virus. Supportive care for clinical signs is the standard treatment for humans, horses, and birds, which may include anti-inflammatory medications and intravenous fluids. Humans and animals with severe symptoms require intensive care, although euthanasia may be recommended for birds and horses.
Management and Prevention
Specific vaccines against WNV for use in horses exist. There are no specific avian vaccines, but commercial vaccines for horses have been used in captive raptors that may provide some protection; exclusion of mosquitoes from areas where birds are kept is still recommended.
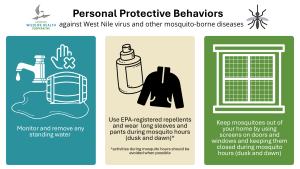
There are no vaccines for people, but personal protective behaviours (see Figure 4) will reduce risk of infection.
Community control measures are applied by some cities in Saskatchewan, such as the application of insecticides to kill mosquito adults and larvae, although pesticide use should be approached with care and there is limited data on its safety and success in reducing WNV cases.
Alternative mosquito control strategies are implemented in other countries. They include using genetically modified mosquitoes, mosquito sterilization/population control programs, and the nature-based Wolbachia method, the latter of which is a famous disease control method applied to dengue-carrying Aedes aegypti mosquitoes by some warm weather cities and countries such as Brazil.
Surveillance
Wild Birds
Because the presence and activity levels of the virus fluctuate depending on the weather, mosquito population, bird population, and the virus’ ability to survive and circulate between them, bird mortalities due to WNV can be (and are) used as a sentinel system to track the disease and anticipate outbreaks. Initial oropharyngeal and cloacal swabs are taken and tested for the virus using the PCR (polymerase chain reaction) method. Shedding of the virus into the environment by these routes depends on stage of disease and the type of bird. Raptors, for example, may shed little to no virus and may be negative on swabs. If WNV remains a suspected cause of disease or death because of observed lesions or history of clinical signs, more sensitive testing is done on tissues.
A 2024 study found that an active surveillance program targeting corvids leads to the detection of WNV before human cases start occurring, provided enough birds are tested.
Mosquitoes
Mosquito surveillance consists of trapping the insects at sites that represent the most likely habitat for WNV-carrying mosquitoes in a specific community. Trapped mosquitoes are then tested for the virus. The level of risk in mosquitoes is determined by using infection rates (number of infected mosquitoes/1000) and a risk index calculation (infection rate*average number of Culex spp. per trap night/1000).
Risk level in an area is determined by combining information including the degree of heat accumulation. Visit the Government of Saskatchewan West Nile Virus Risk Level and Surveillance Results webpage and view the most recent surveillance report for more information.
Implications for wildlife populations
Crows, magpies, and ravens are a very dense and stable population of birds, however, as outbreaks increase in severity (number of deaths) and spread due to climatic changes increased temperatures will lead to longer transmission seasons and allow the disease to spread into new areas), the chance of adverse outcomes for vulnerable wildlife populations increases.

Birds of prey are one such vulnerable group of wildlife; many species are already facing threats such as habitat loss, pollution, climate change, and illegal hunting without taking disease into account. West Nile virus is not the only disease threatening populations either – highly pathogenic avian influenza (HPAI) is another significant cause of avian mortalities. One species susceptible to both WNV and HPAI is the peregrine falcon, which already faced widespread population declines in the mid-20th century but recovered after the ban of organochlorine pesticide DDT and a large-scale captive breeding and reintroduction program that began in the 1970s.
How you can help our disease surveillance efforts
If you find a dead bird or other wildlife, please report it to the regional CWHC office in your area. In Saskatchewan, you can also contact the Western/Northern node by phone at 306.966.5815 or by email at **@*******sf.ca.
In general, risks associated with the collection of animals to determine the cause of death or illness are low if reasonable sanitary precautions are taken. Some animals, however, present more serious concerns than others, such as venomous snakes, bats with potential rabies, and birds at risk for avian influenza. Your regional office can provide advice or guidance if needed. The Canadian Wildlife Health Cooperative has specimen handling instructions on their website.
Written by: Beatriz Garcia de Sousa & Sabine Kirsch, CWHC Western/Northern
Further reading
- Government of Saskatchewan West Nile virus website: West Nile virus overview, when you are at risk, prevention and personal protection, symptoms and severity, information on animal infections
- Government of Canada West Nile virus website: West Nile virus causes, symptoms, risks, treatment, prevention, surveillance, information for health professionals
- National Collaborating Centre for Infectious Diseases West Nile virus website: overview and history of the disease, thorough information on the transmission cycle and rare transmission pathways, explanation of diagnosis process
- Information on Canada’s National West Nile Virus Surveillance System (journal article)
References
Bergmann, F., Fischer, D., Fischer, L., Maisch, H., Risch, T., Dreyer, S., Sadeghi, B., Geelhaar, D., Grund, L., Merz, S., Groschup, M. H., & Ziegler, U. (2023). Vaccination of zoo birds against West Nile virus—a field study. Vaccines, 11(3), 652. https://doi.org/10.3390/vaccines11030652
Bertram, F.-M., Thompson, P. N., & Venter, M. (2020). Epidemiology and clinical presentation of West Nile virus infection in horses in South Africa, 2016–2017. Pathogens, 10(1), 20. https://doi.org/10.3390/pathogens10010020
Castillo-Olivares, J., & Wood, J. (2004). West Nile virus infection of horses. Veterinary Research, 35(4), 467–483. https://doi.org/10.1051/vetres:2004022
de Souza, W. M., & Weaver, S. C. (2024). Effects of climate change and human activities on vector-borne diseases. Nature Reviews Microbiology, 22(8), 476–491. https://doi.org/10.1038/s41579-024-01026-0
Gould, C. V., Staples, J. E., Guagliardo, S. A., Martin, S. W., Lyons, S., Hills, S. L., Nett, R. J., & Petersen, L. R. (2025). West Nile virus. JAMA, 334(7), 618–628. https://doi.org/10.1001/jama.2025.8737
Jackson, A. (2025, July 23). Brazil Opens the World’s Largest Mosquito Biofactory. World Mosquito Program. https://www.worldmosquitoprogram.org/news-stories/brazil-opens-worlds-largest-mosquito-biofactory
Marra, P. P., Griffing, S., Caffrey, C., Kilpatrick, A. M., McLean, R., Brand, C., Saito, E., Dupuis, A. P., Kramer, L., & Novak, R. (2004). West Nile Virus and Wildlife. BioScience, 54(5), 393–402. https://doi.org/10.1641/0006-3568(2004)054[0393:wnvaw]2.0.co;2
McKenzie, M. (2013, November 30). Resurgence of the Peregrine Falcon . Canadian Geographic. https://canadiangeographic.ca/articles/resurgence-of-the-peregrine-falcon/
Mosquito Control Program. Weyburn, Saskatchewan. (2019, January 2). https://www.weyburn.ca/mosquito-control-program/
Tamba, M., Bonilauri, P., Galletti, G., Casadei, G., Santi, A., Rossi, A., & Calzolari, M. (2024). West Nile virus surveillance using Sentinel Birds: Results of eleven years of testing in Corvids in a region of northern Italy. Frontiers in Veterinary Science, 11. https://doi.org/10.3389/fvets.2024.1407271
West Nile Virus Risk Level and Surveillance Results. Government of Saskatchewan. (n.d.). https://www.saskatchewan.ca/residents/health/diseases-and-conditions/west-nile-virus/west-nile-virus-risk-level-and-surveillance-results
West Nile virus. National Collaborating Centre for Infectious Diseases. (2025, July 10). https://www.nccid.ca/debrief/west-nile-virus/
West Nile virus. World Health Organization. (2017, October 3). https://www.who.int/news-room/fact-sheets/detail/west-nile-virus

